
What is Ion Exchange Resin?
Welcome to the World of Ion Exchange Resins
Ion exchange materials are essential for water treatment, chemical separation, and purification processes. These insoluble substances contain loosely held ions that can be exchanged with other ions in solution without altering their physical structure.
Ion exchangers are categorized as cation exchangers (for positively charged ions) or anion exchangers (for negatively charged ions). They are typically insoluble acids or bases with salts of limited solubility, enabling selective ion exchange.
- Highly selective ion removal for precise purification.
- Versatile applications in water treatment, pharmaceuticals, and more.
- Naturally occurring in substances like proteins, cellulose, and soil.
Natural ion exchange materials, such as proteins, cellulose, living cells, and soil particles, play vital roles, like nutrient uptake in plants.


What is Ion Exchange Resin Made Of?
Ion exchange resin is a synthetic organic substance with a solid matrix containing exchange sites. To specify a resin, four key pieces of information are needed:
- Matrix: The solid backbone, e.g., Styrenic, Acrylic, or Phenol Formaldehyde.
- Functional Groups: Chemical groups like Sulphonic, Quaternary Ammonium, or Carboxylic.
- Ionic Form: Exchangeable ions, e.g., H^+, Na^+, Cl^-, or OH^-.
- Type/Structure: Physical structure, such as Isoporous, Macroporous, or Gel.
These components determine the resin’s ion exchange capabilities, making it effective for purification.
How Ion Exchange Resins Soften Hard Water
Understanding Hard Water
Hard water contains high levels of calcium (Ca^{2+}) and magnesium (Mg^{2+}) ions, causing scaling in pipes, interfering with soap lathering, and leaving a metallic taste or dry skin. Hardness can be temporary (removable by boiling) or permanent (requiring chemical treatment).
Permanent hardness, caused by calcium and magnesium salts, can be treated with washing soda to form insoluble carbonates, which are filtered out.

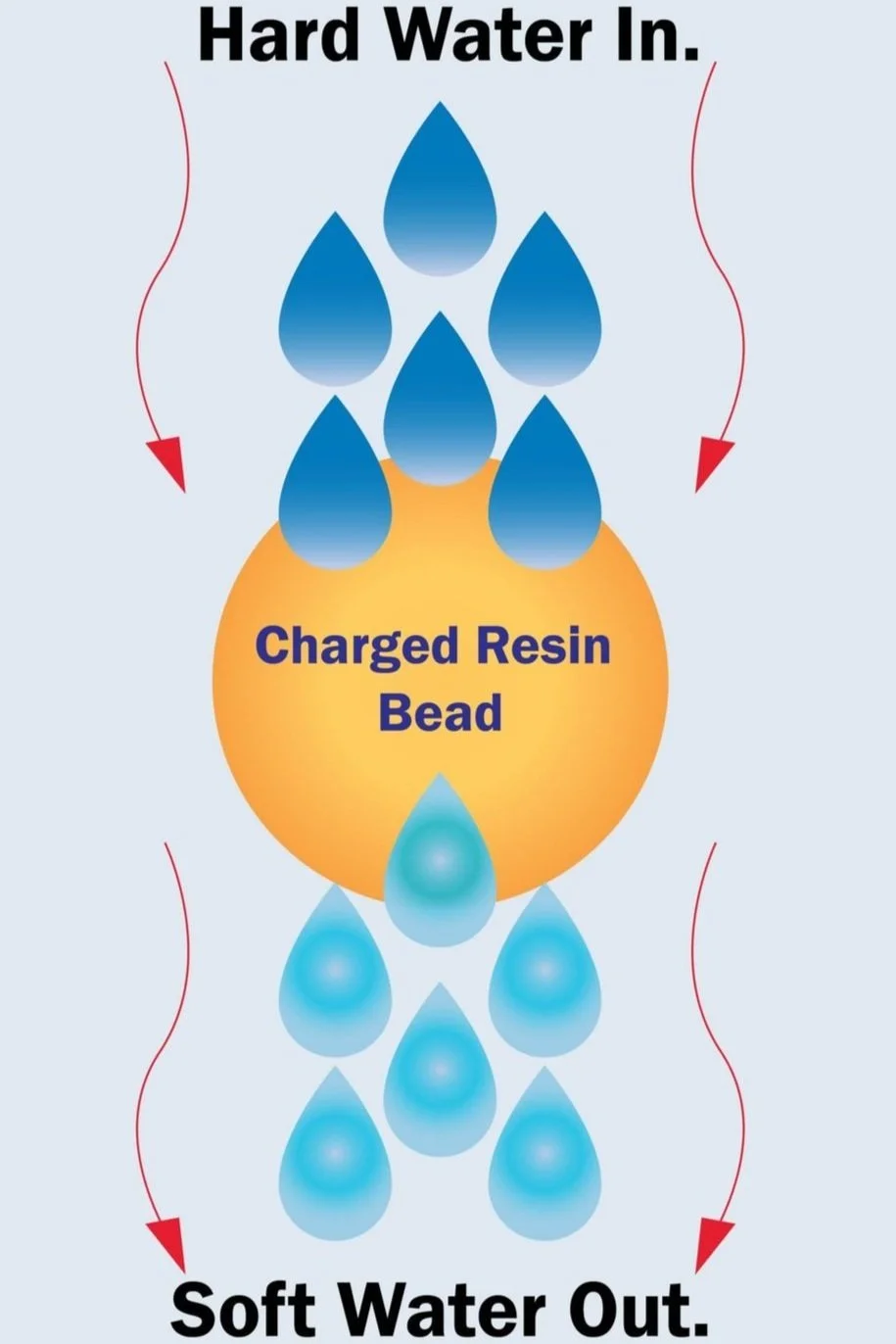
Softening Process
In the softening process, hard water passes through a sodium-form ion exchange resin (Na^+). Calcium and magnesium ions are exchanged for sodium ions, converting hard water into soft water, reducing scaling, and improving water quality.
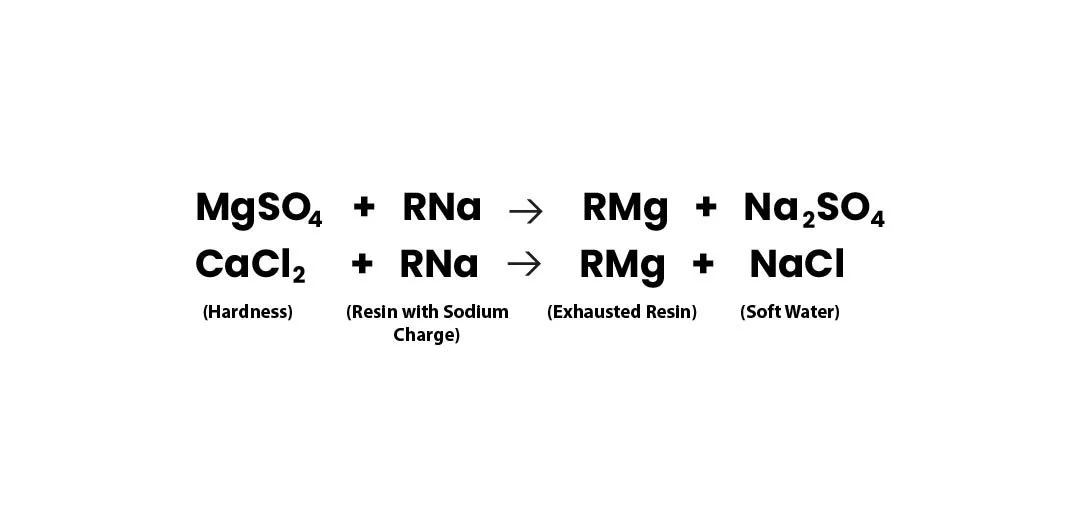
Softening Reaction:
2RNa + CaCl₂ → R₂Ca + 2NaCl 2RNa + MgSO₄ → R₂Mg + Na₂SO₄
Regeneration of Softening Resins
When the resin becomes saturated with Ca^{2+} and Mg^{2+} ions, it is regenerated using a brine solution (NaCl). Sodium ions displace the hardness ions, restoring the resin’s capacity.
Regeneration Reaction:
R₂Ca + 2NaCl → 2RNa + CaCl₂ R₂Mg + 2NaCl → 2RNa + MgCl₂
Applications of Ion Exchange Resins
Water Softening
Removes calcium and magnesium ions to prevent scaling and improve water quality.
Dealkalization
Reduces water alkalinity to lower hardness and sodium content.
Demineralization
Completely removes minerals for high-purity water in industrial applications.
Conclusion
Ion exchange resins are a cornerstone of modern purification, offering efficient solutions for water softening, dealkalization, and demineralization. With Doshion’s advanced resin solutions, industries and households can achieve reliable, high-quality water treatment.
Explore Doshion’s industrial water treatment resins for transformative solutions. Visit Doshion's Industrial Water Treatment Resins page to learn more.