
Water Chemistry: Importance in Wastewater Treatment
Water Chemistry
14 Oct 2025 | Water Treatment & Environmental Science
Water is essential for life, being the most abundant mineral on Earth's surface and a major component of all living matter, accounting for approximately 75\% of its composition. In its pure state, water is a colorless, odorless, and tasteless liquid, but its solvent properties allow it to dissolve many substances, introducing impurities in natural sources.
Only 0.1\% of Earth's water is available as freshwater, with annual per capita consumption averaging 250 \, \text{m}^3. This scarce resource faces threats from pollution and unsustainable development, making its protection, careful use, and recycling critical.

Why is Wastewater Treatment Essential?

Sewage treatment, or wastewater treatment, purifies water for reuse or safe environmental release by removing impurities. Untreated wastewater contains high levels of organic material, pathogenic microorganisms, nutrients, and toxic compounds, posing risks to human health, aquatic ecosystems, and waterways.
Modern wastewater treatment views wastewater as a resource for energy, nutrients, and water for industrial, agricultural, and even potable uses.
Key Reasons for Wastewater Treatment
- Removal of physical, chemical, and biological contaminants
- Protection of aquatic ecosystems
- Reduction of waterborne diseases
- Preservation of water resources for reuse
Water Structure and Polarity
Water’s molecular structure consists of one oxygen atom bonded to two hydrogen atoms, forming a bent geometry. Oxygen’s two lone pairs create a partial negative charge, while the hydrogen atoms carry a partial positive charge due to electronegativity differences, making water a polar molecule. This polarity underpins its role as a universal solvent and influences its physical properties.
Important Properties of Water

Density
Pure water has a density of 1.0 \, \text{g/cm}^3 at 4^\circ\text{C}. Seawater, with a salinity of 3.5 g/L, has a density of 1.028 \, \text{kg/L} at 0^\circ\text{C}.
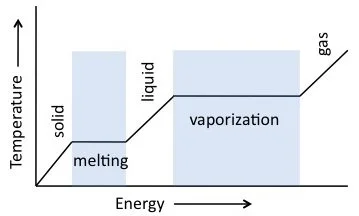
Thermal Properties
Water’s specific heat is 1 \, \text{kcal/kg} at 0^\circ\text{C}, with a latent heat of fusion of 79 \, \text{kcal/kg} and vaporization of 539 \, \text{kcal/kg}, making it ideal for heat transfer.
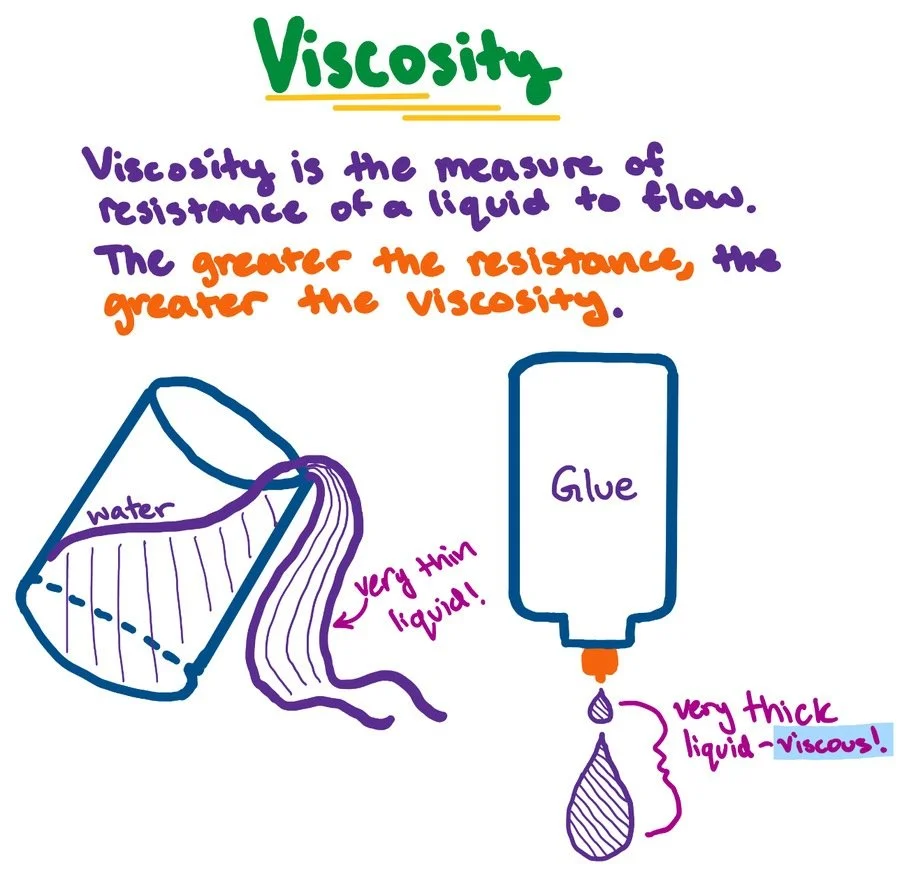
Viscosity
Viscosity, which resists flow, decreases with temperature and increases with dissolved salts, affecting head loss in water treatment.
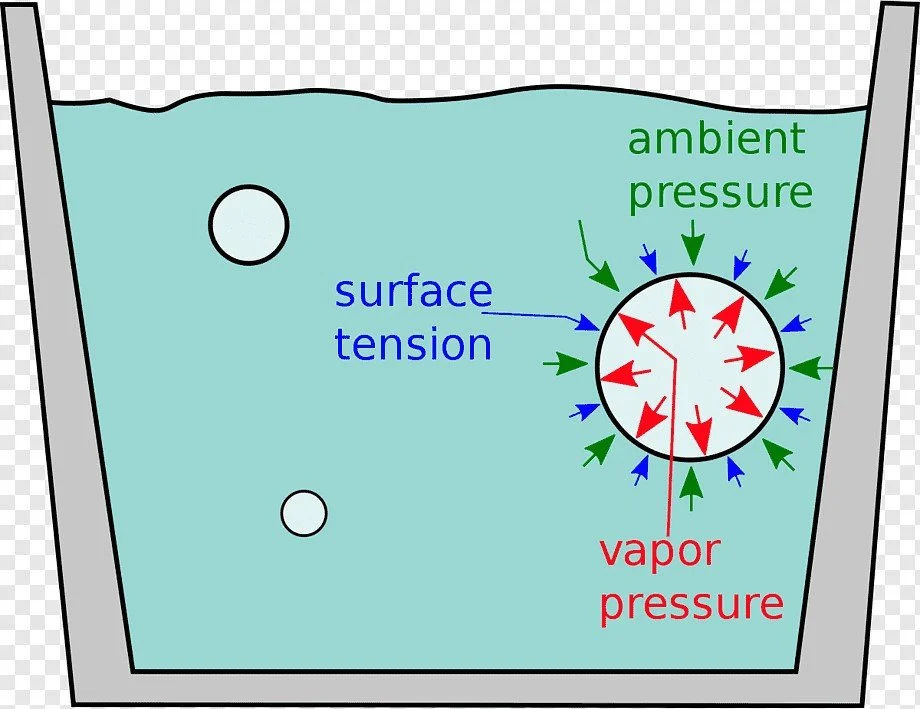
Surface Tension
Surface tension, driven by cohesive forces, decreases with temperature and increases with dissolved salts, impacting treatment processes.
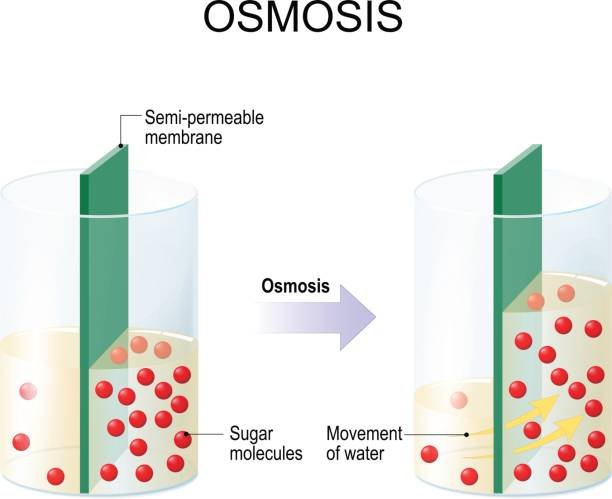
Osmotic Pressure
Osmotic pressure, given by \Pi = \Delta C R T, is critical for reverse osmosis in water treatment.

Electrical Conductivity
Pure water has a conductivity of 0.037 \, \mu\text{S/cm}, influenced by dissolved ions.

Compressibility
Water’s low compressibility (1.8% volume reduction in deep oceans) allows effective pressure transmission.
Boiling and Freezing Points
Water freezes at 0^\circ\text{C} and boils at 100^\circ\text{C}, critical for environmental and industrial applications.
Water Chemistry in Wastewater Treatment: The Essential Link
The chemical properties of water are the driving forces behind effective wastewater treatment. Understanding parameters like \text{pH}, dissolved oxygen, and contaminant nature is crucial for optimizing physical, chemical, and biological treatment processes.
Critical Chemical Parameters
- \text{pH} (Acidity/Alkalinity): Optimal range (6.5–7.5) for biological processes; critical for chemical coagulation.
- Dissolved Oxygen (DO): Essential for aerobic digestion to reduce \text{BOD}.
- Oxidation-Reduction Potential (ORP): Controls nitrification/denitrification and disinfection.
- Alkalinity: Neutralizes acids in nitrification, preventing \text{pH} crashes.

Physical Properties in Process Design
| Property | Application in Wastewater Treatment |
|---|---|
| Density | Influences sedimentation (heavier particles settle in clarifiers) and flotation (lighter particles rise). |
| Viscosity | Affects mixing energy in aeration tanks and flow through membranes/filters. |
| Surface Tension | Critical for foam formation in aeration basins, signaling surfactant issues. |
Water chemistry provides the data needed to manage treatment reactions, ensuring effluent meets environmental discharge standards.