
How Does Ion Exchange Resins Work?

How Does Ion Exchange Resins Work?
Published on 14 Nov 2025
How Ion Exchange Resins Work
Ion exchange resins are vital in industries like water treatment and chemical purification due to their ability to selectively exchange ions in a solution. This process involves transferring ions between the resin and the solution, removing impure ions and replacing them with pure ones, enabling applications like water softening and demineralization.
The operation of an ion exchange system is straightforward. Resins trade charged particles in the solution for different ones in the resin, purifying or altering the solution’s composition. Functional groups, immobile ions bound within the resin’s polymer matrix, attract and bond with oppositely charged ions in the solution. For cation exchange resins, functional groups like sulphonic or carboxylic acids are used, while anion exchange resins use ammonium groups.
During the process, the solution flows through a bed of ion exchange resin beads. The functional groups attract counterions in the solution, displacing existing ions in the resin via electrostatic attraction, achieving purification.
Ion Exchange Chemistry
Cation Resin Reactions
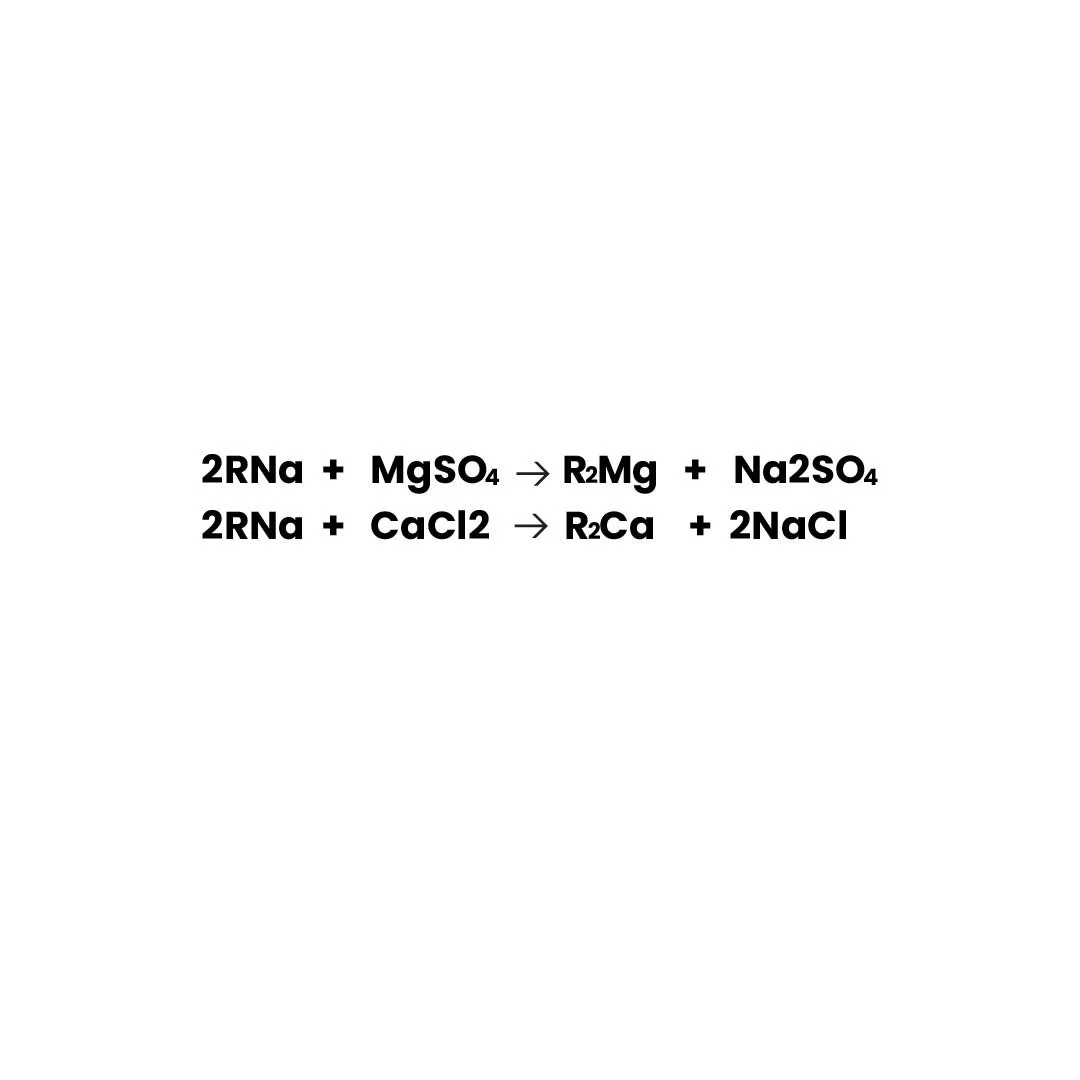
Water containing magnesium (Mg^{2+}) and calcium (Ca^{2+}) salts is purified using sodium-form resin. Impure ions bond with the resin, while sodium ions (Na^+) react with impurities to form sodium sulphate and chloride salts, enhancing water purity.
Reaction Chemistry:
2RNa + MgSO₄ → R₂Mg + Na₂SO₄ 2RNa + CaCl₂ → R₂Ca + 2NaCl
Where, R = Resin
Anion Resin Reactions
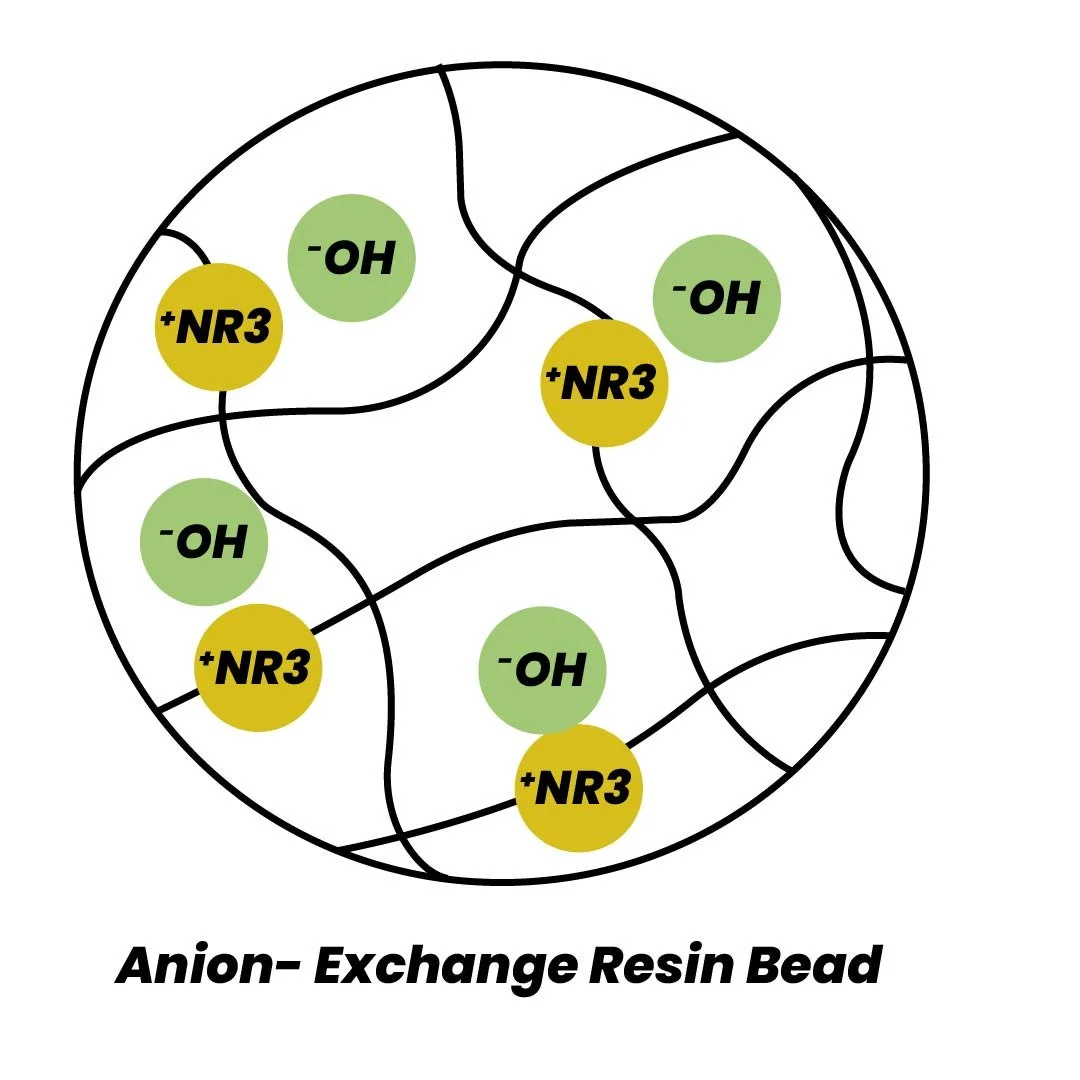
Water with mineral acidity, when passed through resin in hydroxide form (OH^-), undergoes an exchange where impure ions like carbonates and sulphates are replaced by hydroxide ions, producing pristine water.
Reaction Chemistry:
ROH + H₂CO₃ → RCO₃⁻ + H₂O ROH + H₂SO₄ → RSO₄⁻ + H₂O
Where, R = Resin
Regeneration of Resins
As contaminants bind to the resin’s exchange sites, its capacity diminishes. Regeneration reverses the ion exchange using a concentrated regenerant solution—acid for cation resins and base for anion resins—to restore functionality.
Cation Resin Regeneration
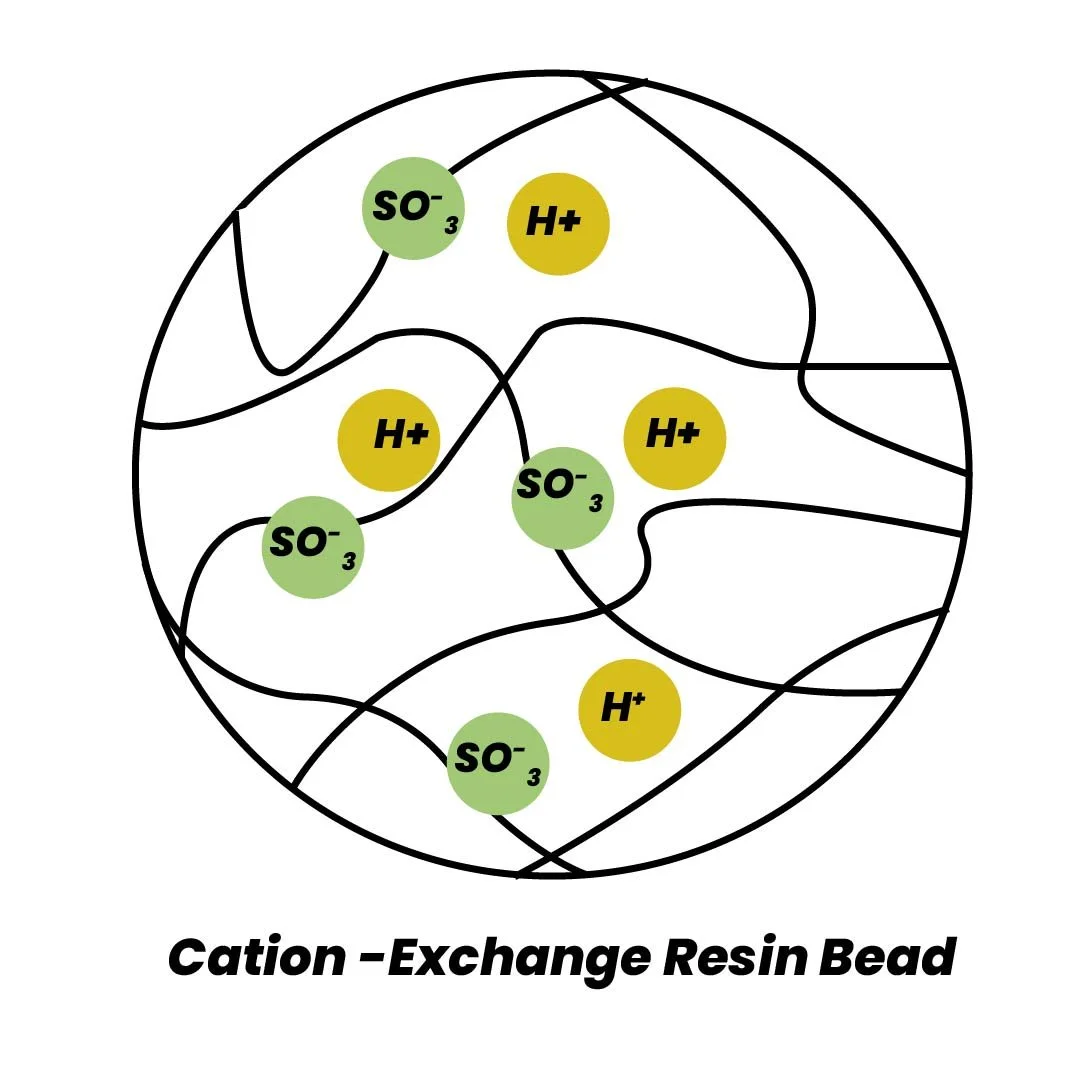
Cation resins are regenerated with an acid solution, such as hydrochloric acid (HCl) or sodium chloride (NaCl), displacing magnesium and calcium ions to restore the resin to its sodium form.
Reaction Chemistry:
R₂Mg + 2NaCl → 2RNa + MgCl₂ R₂Ca + 2NaCl → 2RNa + CaCl₂
Where, R = Resin
Anion Resin Regeneration
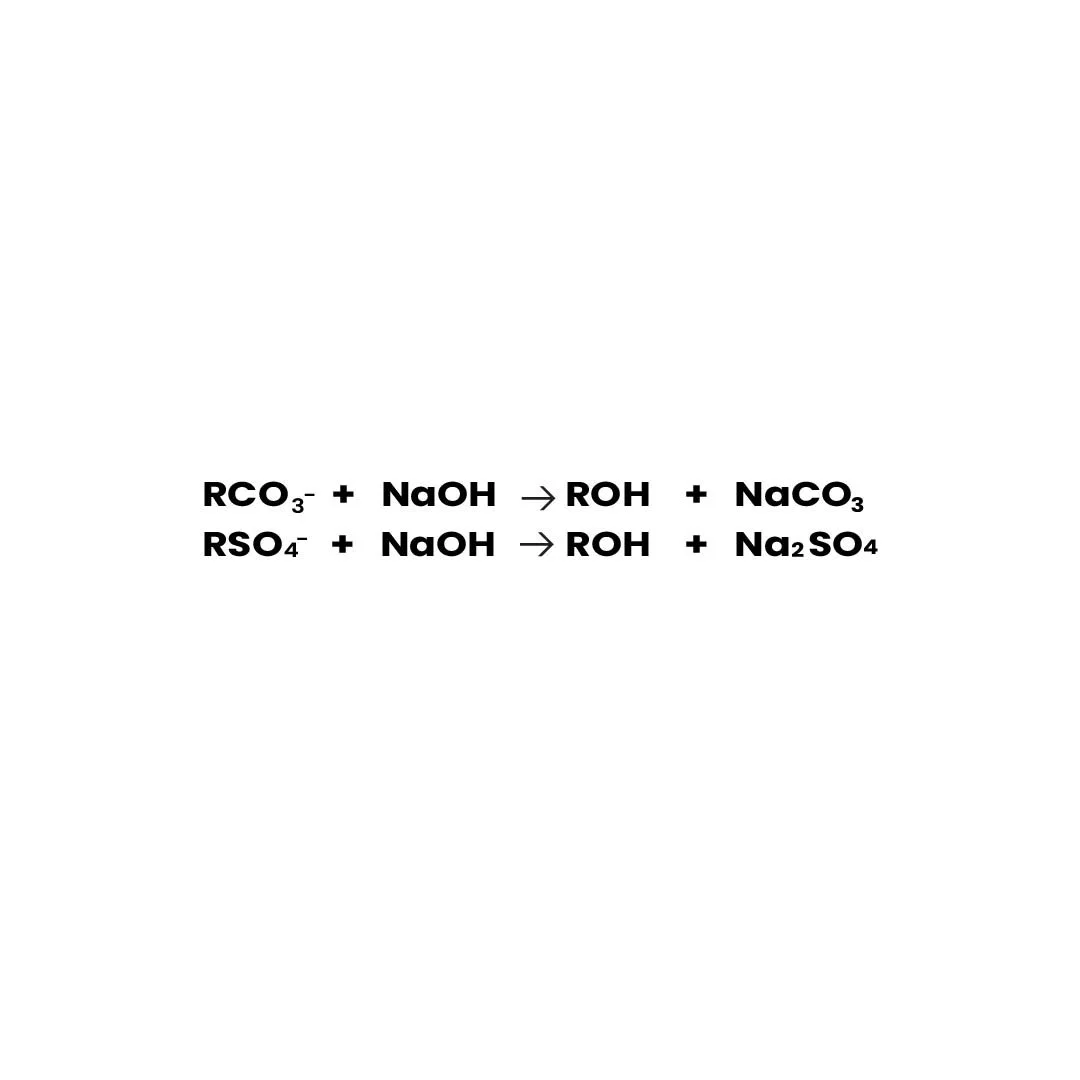
Anion resins are regenerated with a strong base, such as sodium hydroxide (NaOH), displacing captured anions to restore the resin’s hydroxide form.
Reaction Chemistry:
RCO₃⁻ + NaOH → ROH + NaCO₃ RSO₄⁻ + NaOH → ROH + Na₂SO₄
Where, R = Resin
Advantages of Ion Exchange Resins
- Optimal performance with rapid results.
- Highly effective at removing inorganic ions from water.
- Suitable for short- and long-term applications.
- Chemically stable, long-lasting, and easy to regenerate.
- Simple automation and adaptation to unique settings.
- Compact design with excellent treatment capabilities.
- High mitigation efficiency.
- Easy to install and operate.
- Low maintenance requirements.
- Regenerable, extending resin lifespan.
- Cost-effective with low operating and maintenance costs.
- Inexpensive initial investment and regenerant solutions.
Limitations of Ion Exchange Resins
- Significant long-term operational costs for equipment.
- Ineffective at eliminating bacteria from water.
- Effluent disposal can pose environmental risks.
References
Conclusion
Ion exchange resins are a cornerstone of modern water treatment and industrial processes, offering efficient ion removal and water purification. With Doshion’s advanced ion exchange resin solutions, industries can achieve reliable, high-quality results tailored to diverse purification needs.
Explore Doshion’s industrial water treatment resins for transformative solutions. Visit Doshion's Industrial Water Treatment Resins page to learn more.
Contact us today to discover more about our ion exchange resins, pricing, and partnership opportunities.